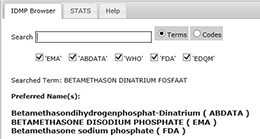
IDMP Term Browser
Matching controlled vocabulary for identification of medicinal products
The overall scope of the IDMP Term Browser
is to support the implementation of the international standards for Identification of Medicinal Products (IDMP) but in the shorter-term it is to support the implementation of the eXtended Eudravigilance Medicinal Product Dictionary (XEVMPD).Who is affected by IDMP?
- Regulatory affairs staff of pharmaceutical companies
- Representatives of IT departments of medicines
- regulatory authorities, pharmaceutical companies, and service providers
- EU Qualified Persons responsible for Pharmacovigilance (EU QPPVs)
- Pharmacovigilance staff of pharmaceutical companies and medicines regulatory authorities
- Medicinal product management software vendors
- Sponsors of clinical trials
EMA and FDA submission codes help you make your IDMP project a success!
You are kindly invited to explore the beta version of the first terminology browser in pharmazie.com for Health Professionals in the eSubmission.