Our IDMP Browser helps you make your IDMP projects a success
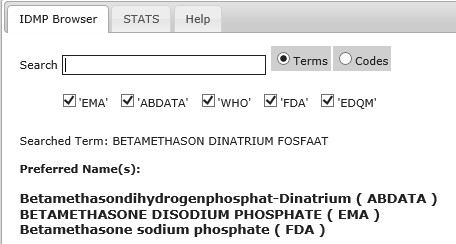
The overall scope of the IDMP Term Browser is to support the implementation of the International Standards for Identification of Medicinal Products (IDMP) but in the shorter-term it is to support the implementation of the eXtended Eudravigilance Medicinal Product Dictionary (XEVMPD).
In a nutshell:
• Matching controlled vocabularies in eSubmission
• Networking international medicinal products
• Cleaning up your databases
• Integrating FDA´s “Structured Product Labeling” (SPL) Terminology
• Integrating EMA´s XEVMPD Terminology
• Integrating EMA´s IDMP Terminology
• Aimed to assist you in the journey from XEVMPD to IDMP
You may subscribe for a test account and download the IDMP Term Browser brochure which navigates the complexities of the controlled vocabularies in XEVMPD and IDMP.
Get a Test-Account
[pharmazie.com] is not only working to ensure you have all the information you need for coding your products according to the rules, but also working to assist you in the step from XEVMPD to IDMP. To learn more about our services in managing IDMP´s controlled vocabulary in your database, download our IDMP service brochure.
Please feel free to contact us with any questions you may have, or if you are interested in seeing a demo of the IDMP Term Browser.

